fMRI provides noninvasive 3D imaging of brain activity using blood oxygen level dependent (BOLD) or cerebral volume (CBV) dependent functional MRI with high spatial resolution. The Gozzi lab has established a set of methods for reliably mapping resting state fMRI (rsfMRI) networks in the lightly anesthetized and awake head-restrained mouse. These measurements are carried out on dedicated a 7T small bore animal scanner equipped with room temperature and cryo-refrigerated receive coils. 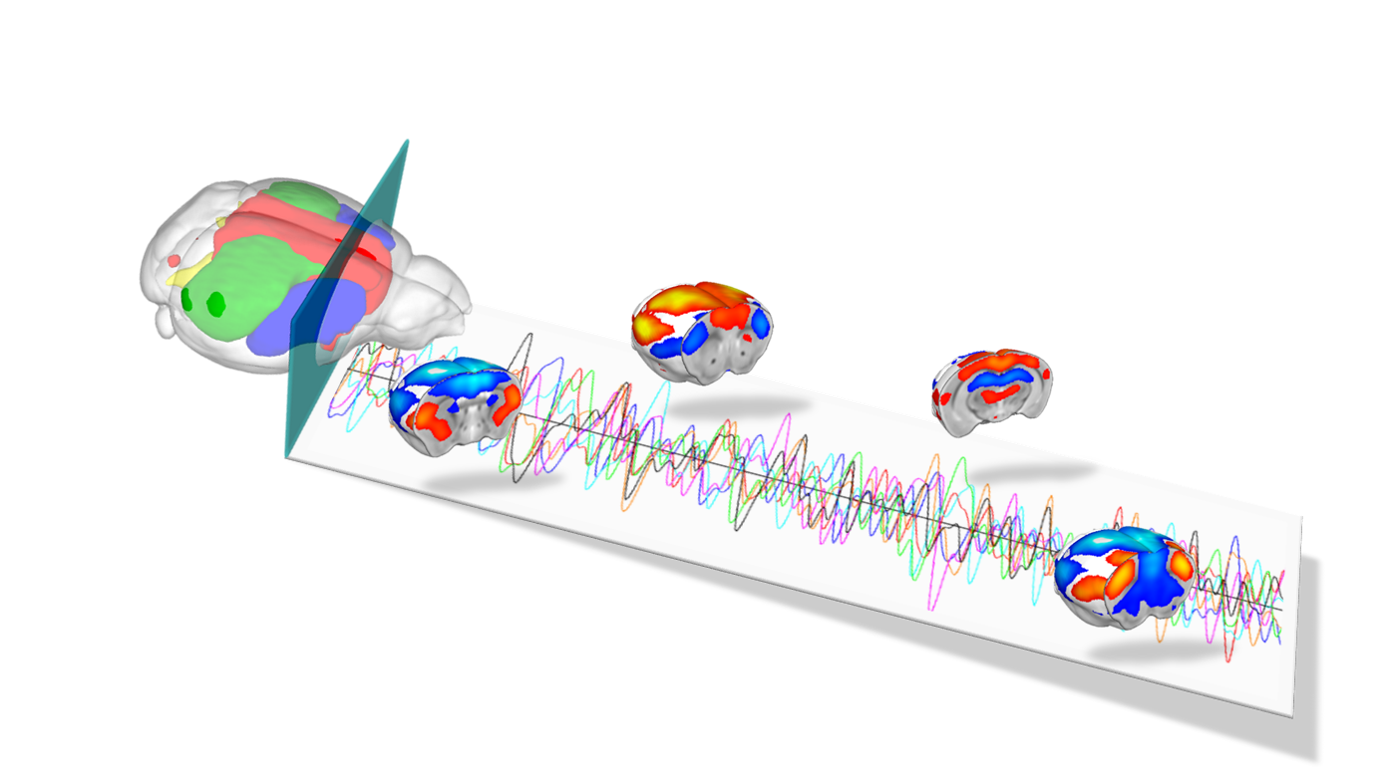
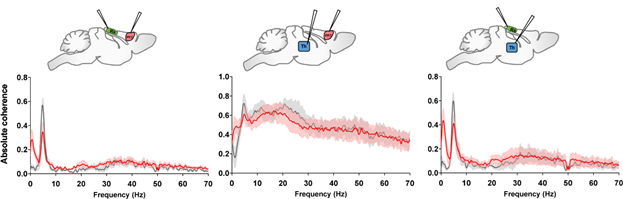
Electrophysiology records the electrical impulses produced by the neurons in the brain to offer insight into changes of activity states and connectivity between regions. In the Gozzi lab, in vivo multielectrode electrophysiological recordings are used to map firing of individual neurons in response to neural maniupulations, and the summation of their electrical activity into oscillations. which can indicate different states. These measurements are coupled to resting state or evoked fMRI recordings to gain information about how local neural activity propagates at the network level. The lab is currently equipped to record extracellular activity using high channel count multielectrode arrays in lightly anesthetised mice and in head fixed awake mice.
Chemogenetics is a neural platform entailing the expression of engineered G-protein coupled receptors to remotely and non-invasively modulate neural activity in the living brain. Our lab employs “designer receptors exclusively activated by designer drugs” (DREADDs) to increase or inhibit neuronal function in specific cell-types and regions of the mouse brain. The combined use of chemogenetics and fMRI (chemo-fMRI) permits to establish causal links between regional brain activity and brain-wide functional connectivity, hence providing a powerful bridge between different levels of inquiry in experimental neuroscience. 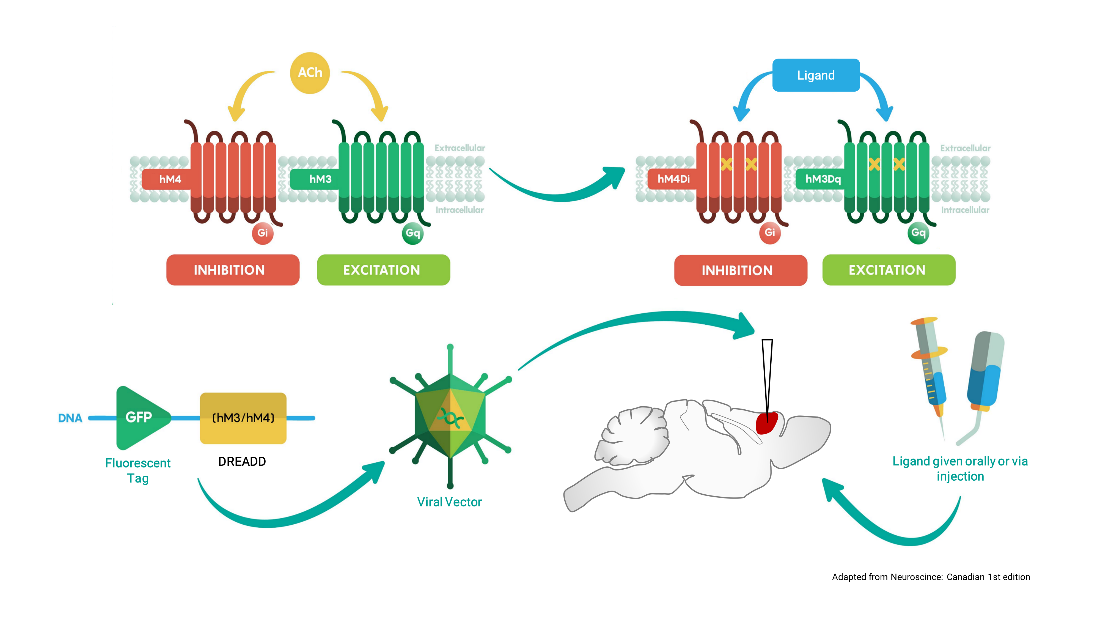
Optogenetics allows precise spatio-temporal control of neural activity via the illumination of neuronal channels (opsins) engineered to be light sensitive. Regionally-targeted manipulations of neuronal function in vivo can therefore be attained via surgically implanted optical fiber. In collaboration with the Pisanello lab at the IIT, optogenetic-fMRI studies in the lab entail the use of tapered optical fibers. This strategy allows to homogeneously illuminate large sections of tissue for network level manipulations of neuronal function with fewer artefacts than traditional flat fibers. 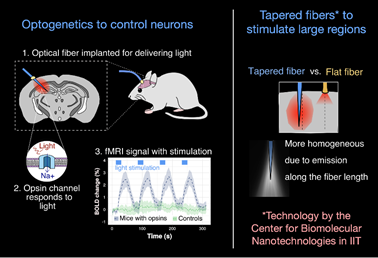
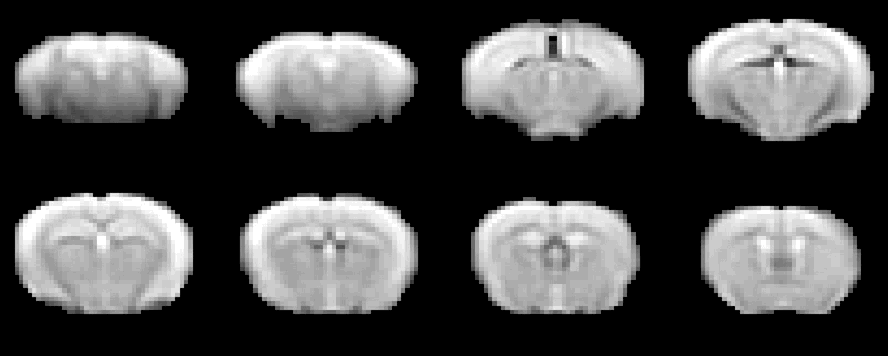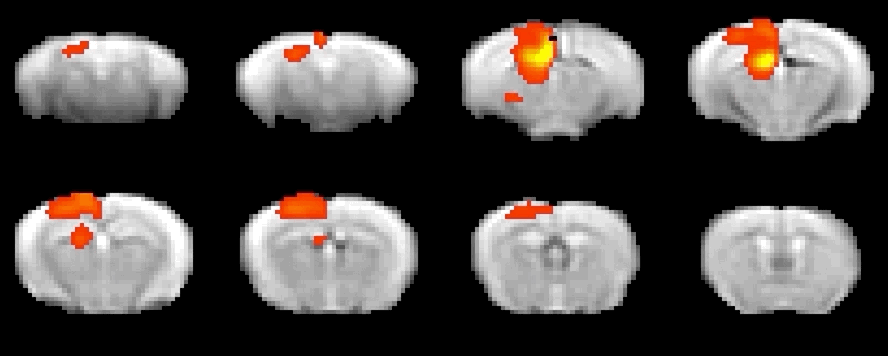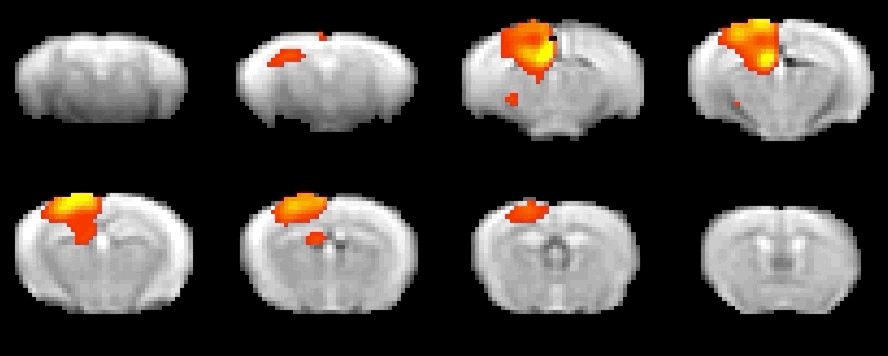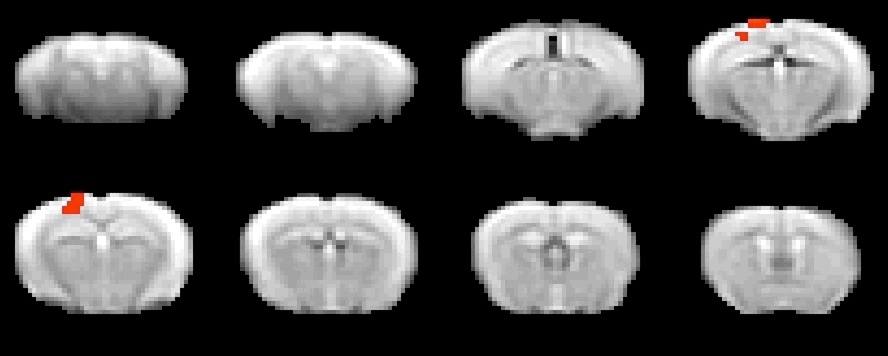
To investigate local neural and neurotransmitter activity at rest or in freely behaving animals, we employ fiber photometry. This method uses optic fibers, implanted in the mouse brain to detect bulk-changes in calcium dependent fluorescence of neurons which are genetically tagged. This technique is well suited to monitor neural population activity in the cortex, or deep nuclei. With the use of novel viruses, we can also monitor specific neurotransmitter activity, leveraging the same fluorescence mechanism. The laboratory has a dual-site photometry equipment (Doric Lenses) with specialized hardware and software (TDT), allowing fiber photometry to be performed within the MRI scanner.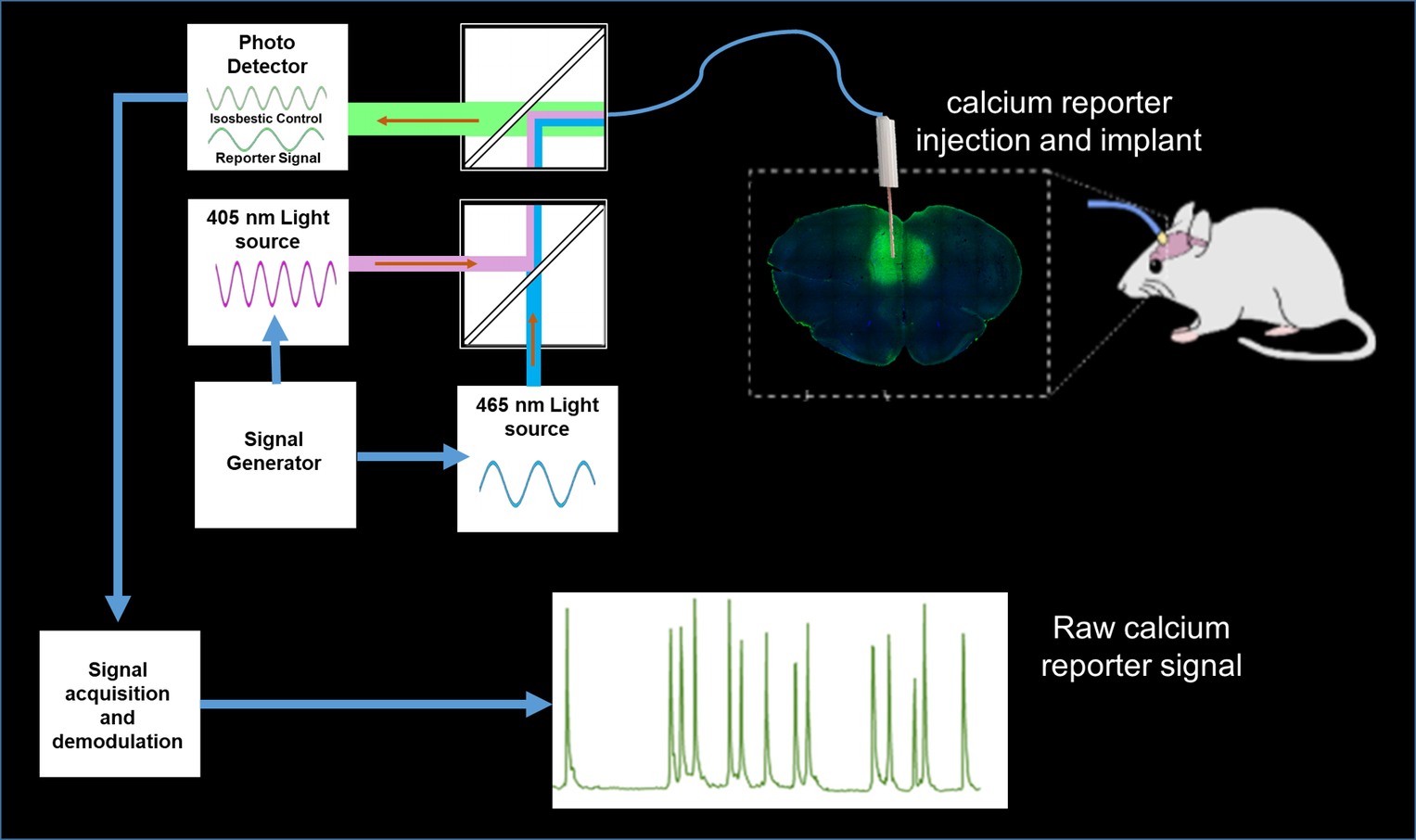
An important goal of our research is to assess how altered functional connectivity can bias behavioural output. To this aim, we have established a number of assays aimed to probe core behavioural traits relevant for the investigation of autism and related neurodevelopmental disorders (i.e. learning, memory, social interaction, motor stereotypies, reward processing etc.). These are routinely probed in a number of different tests and settings, including the three-chambered social test, habituation-dishabituation test, social conditioning place preference test, t-maze, t-maze, open field, object recognition test.